Which of the Following Is a Covalent Molecule
The bond between the O atom and one of the H atoms in the same molecule is a covalent bond. The transfer of electrons from the metal to the non-metal.

Co Ordinate Covalent Bond Definition In Chemistry Covalent Bonding Chemistry Lessons Chemistry
An ionic bond between oppositely charge ions.

. The octet is incomplete because Be has 2 valence electrons of its own and after bonding with 2Cl atoms it gains two more electrons. Break up into ions. Which of the following compounds would NOT have covalent bonds.
Double Bond in Ethylene Molecule. O2 Molecule with Double Covalent bond. These are usually formed when a metal reacts.
The number of electrons lossed or gained by each ion. Which of the following statements contrasting covalent bonds and ionic bonds is correct. CH 3 CH 2 CH 3 B.
Take note of they only occur between nonmetals. O 2 Covalent bond between O atoms. Due to weak intermolecular forces they are easy to distort or break.
Which of the following is a nonpolar covalent molecule. Have low melting point D. The bond between one water molecule and a second water molecule is a covalent bond.
1 Which pair of elements is most apt to form a molecular compound with each other. All of the above. PH 3 Covalent bond between P and H.
Hence its octet is incomplete. Thus it is not a covalent molecule. Ionic bonds consist of metal and nonmetal elements.
Nitrogen dioxide NO 2 is a reddish-brown toxic gas that is a prominent air pollutant produced by internal combustion engines. In a polar covalent bond the more electronegative atom has a ____ charge. A MnCl2 Ob K3N O c Co₂53 Od BrF3 Question 12 3 points A covalent bond is best described as.
These elements are all nonmetals and are therefore a covalent bond. So if two identical nonmetals eg two hydrogen atoms bond together they will form a pure covalent bond. A slightly negative B slightly positive C neutral D.
Water molecules contain covalent bonds. In ethylene each carbon atom shares two of its valence electron with two hydrogen atoms and remaining two electrons with the other carbon atom. The transfer of electrons from the metal to the non-metal.
Diamond for example consists of carbon atoms held together by covalent bonds in a crystalline structure. H 2 SO 4 Covalent bond between S and O and also between O and. The bond between the O atom in one molecule and one of the H atoms in another molecule is a covalent bond.
Have high boiling points Correct Answer. Which of the following properties of covalent compounds is not correct. A NO2 B N2O4 C H2O2 D K2O E all.
On the other hand covalent bonds consist of nonmetal elements only. Which statement best describes a covalent bond. Do not break up into ions.
A the result of the transfer of electrons from one atom to another. Covalent molecular compounds do not conduct electricity because they _____ answer choices. The molecular formula of octane is C 8 H 18.
The intermolecular attractions are weak which require a small amount of energy. Which of the following is a covalent molecule 1 H2O2 NH33 HCL4 All. So there is a double bond between the carbon atoms.
Table sugar consists of hydrogen oxygen and carbon. Option D Explanation No official explanation is available for this question at this time. Correct option is A In BeCl 2.
Oc a bond between a metal and a nonmetal. When two dissimilar nonmetals form bonds eg hydrogen and oxygen they will form a covalent bond but the electrons will spend more time closer to. A n _____ is the smallest particle of a covalent compound that still has the properties of the compound.
A chemical bond that involves sharing a pair of electrons between atoms in a molecule. Are mostly gaseous and volatile liquids C. Covalent compound is defined as the compound which is formed by the sharing of electrons between the atoms forming a compound.
Hence it has a total of 4 electrons which is less than 8. B 2 H 6 Covalent bond between B and H atoms. Mg is a metal while Cl is a nonmetal.
Note that network solids are compounds containing covalent bonds that violate some of these rules. Which of the following has the highest boiling point. N-F A I only B II only C III only D I and III E I II and III.
Do not dissolve in water. Please check contributions posted by. A covalent molecule exists when two nonmetals form a bond by sharing valence electrons.
Lets analyze the given choices. Examples of molecular compounds that dont dissolve well in water are oil and polymerized plastic. The molecule among the following that exhibits resonance structures is A CO2 B CH4 C SO3 D NH3.
These are usually formed when two non-metals react. Is composed of magnesium Mg and chlorine Cl. Which of the following is a molecular covalent compound.
Is composed of potassium K and sulfur S. Which of the following statements about water is not correct. Low melting points and boiling points.
The first element in the formula is simply listed using the name. 2 KCl Ionic bond between K and Cl. An ionic compound is defined as the compound which is formed when electron gets transferred from one atom to another atom.
Solids are brittle and variously coloured. Naming binary two-element molecular compounds is similar to naming simple ionic compounds. Following are the properties of covalent compounds.
H 2 O D. Ob the result of the sharing of electrons between atoms. A aluminum oxygen B magnesium iodine C sulfur fluorine D potassium lithium E barium bromine 2 Which of the following bonds would be best categorized as covalent.
An ionic bond between oppositely charge ions. Superior malleability is not a property of covalent compounds. Covalent bonds form when two nonmetallic atoms have the same or similar electronegativity values.
The bond between two H atoms in the same molecule is a covalent bond. Find an answer to your question Which of the following is an example of a covalent compound. See what the community says and unlock a badge.
A covalent bond that has an unequal sharing of electrons and the electronegativity difference is.

Common Examples Of Covalent Compounds Covalent Bonding Chemical Bond Molecules

Covalent Bonding Scribble Notes Covalent Bonding Middle School Science Teacher Middle School Science Classroom

Ionic Covalent Bonding 28 Chemistry Task Cards Covalent Bonding Task Cards Chemistry Classroom

Covalent Compound Lewis Structure Worksheets Teaching Chemistry Chemistry Classroom Chemistry Lessons

Lewis Dot Structures Covalent Bonding Student Learning Octet Rule

Ionic Covalent And Metallic Bonds Chemical Bonds Chemistry Khan Academy Chemical Bond Chemistry Teaching Chemistry

Pin By Wan M On Og Wan Covalent Bonding Molecular Chemistry

Pin By Playmada Games On Collisions Chemistry Covalent Bonding Covalent Bonding Element Symbols Molecular Shapes

Ionic Vs Covalent Bonds Chemistry Notes Chemistry Chemistry Education

Covalent Compounds Examples And Properties Covalent Bonding Ionic And Covalent Bonds Ionic Compound

Nomenclature Worksheets 3 Covalent Molecular Compounds Answer Key Chemistry Lessons Teaching Chemistry Chemistry Classroom
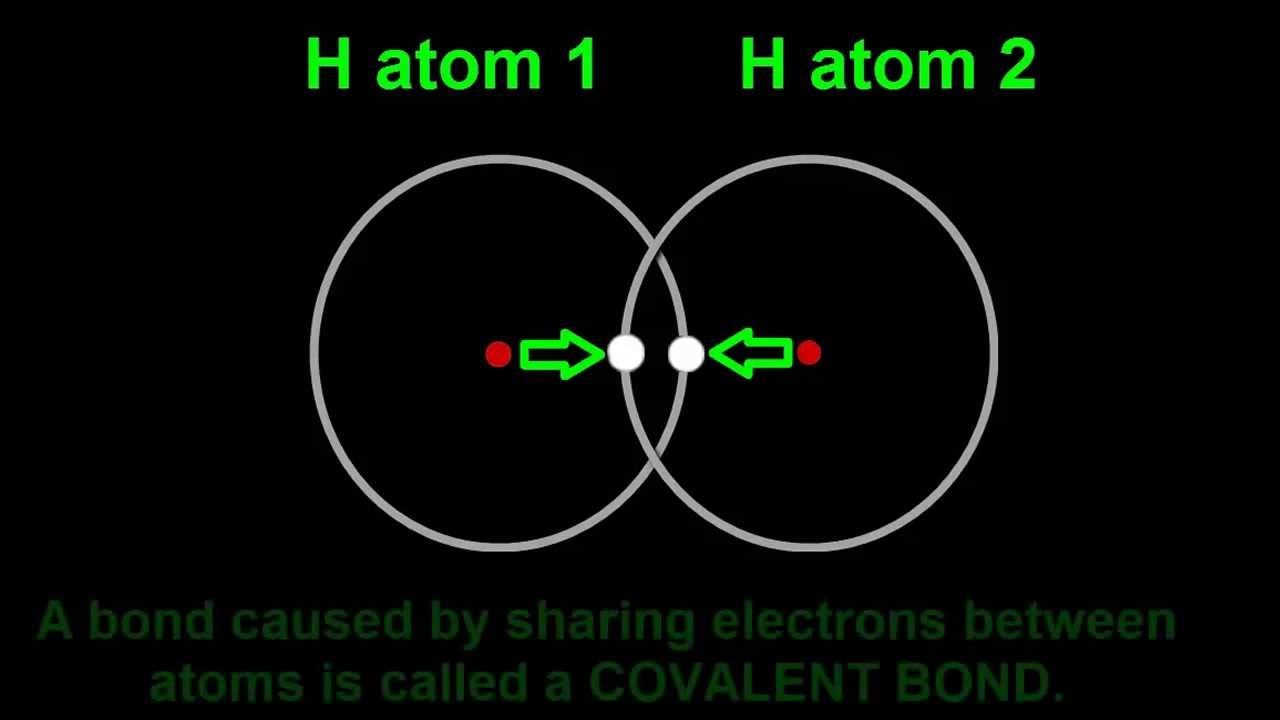
How Two Hydrogen Atoms Join To Become A Hydrogen Molecule H2 Covalent Bonding Molecules Hydrogen Atom

Covalent Bonding Worksheet Answer Key Covalent Bonding And Vsepr Theory Worksheet Covalent Bonding Worksheet Covalent Bonding Super Teacher Worksheets

Learn The Difference Between Ionic And Covalent Bonds See Examples Of The Two Types Of Ch Ionic And Covalent Bonds Covalent Bonding Covalent Bonding Worksheet

Lewis Dot Structures Of Ionic And Covalent Compounds Chemistry Worksheets Persuasive Writing Prompts Covalent Bonding Worksheet

Difference Between Covalent And Ionic Bonds Ionic Bonding Chemical Bond Chemistry Classroom

Learn The Difference Between Ionic And Covalent Bonds See Examples Of The Two Types Of Ch Ionic And Covalent Bonds Covalent Bonding Covalent Bonding Worksheet


Comments
Post a Comment